The colour purple - no passing off in Glaxo v Sandoz
The England and Wales High Court has recently rejected a claim by Glaxo against Sandoz relating to use of the colour purple for inhalers to treat asthma and chronic obstructive pulmonary disease (COPD).
Background
Glaxo markets a combination of salmeterol and fluticasone for the treatment of asthma and COPD under the trade mark Seretide, in two different types of inhaler branded Accuhaler and Evohaler, which are coloured shades of purple. In 2010 and 2011 Seretide had over 42 per cent of the UK inhaler market and current UK sales still exceed £400 million per annum. From 1999 to May 2015, the Seretide Accuhaler and Evohaler were the only inhalers on the UK market which were coloured purple. In 2015, Sandoz launched a branded generic competitor to the Seretide Accuhaler called the AirfluSal Forspiro, which was also purple. Glaxo brought an action before the court claiming that Sandoz had passed off the AirFluSal Forspiro as being (i) connected in the course of trade with Glaxo and/or (ii) equivalent to the Seretide Accuhaler by virtue of its get-up and packaging, as a result of use of the colour purple.
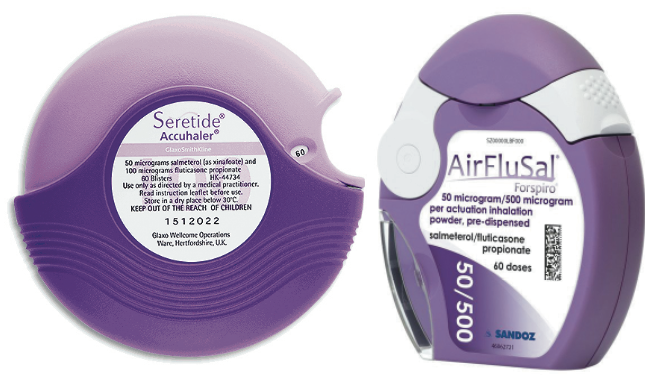
Decision of the court - preliminary points
Both the Seretide Accuhaler and the AirFluSal Forspiro are prescription-only medicines, which are not generally marketed directly to patients. Prescriptions are increasingly written by brand name and not generically.
Initially, Glaxo argued that the deception as to trade origin impacted both healthcare professionals and patients themselves, but by trial this was confined to the issue of deception of patients only.
There was some common ground between the parties that informal colour conventions had arisen in relation to the treatment of asthma in the UK, for example, use of the colour blue for SABA relievers. There was a safety element to this because patients often had more than one type of inhaler and needed to be able to quickly tell them apart. Sandoz also argued that it was common practice for generics to adopt similar colour schemes to the originator products, as this promoted familiarity amongst patients and adherence to drug regimes.
The AirFluSal Forspiro was not licensed for asthma (only COPD) until 2017.
Decision of the court - passing off: connected in course of trade with Glaxo due to use of colour purple
The judge considered that Glaxo had failed to demonstrate that in 2015 the colour purple was distinctive of the trade origin of Seretide in the mind of the relevant public. The judge was persuaded by (i) the presence of other generic salmeterol/fluticasone combination inhalers on the market which were purple and which were apparently unchallenged by Glaxo, (ii) the informal colour conventions which existed in relation to inhalers in the UK and (iii) the fact that there was no evidence of any confusion or deception suffered by any patients since 2015.
Decision of the court - passing off: equivalent to Seretide Accuhaler due to use of colour purple
The judge found that Glaxo had failed to demonstrate that in 2015 the colour purple was distinctive of the relevant characteristics of the Seretide Accuhaler.
The judge considered (i) the fact that Glaxo itself used different shades of purple for different strengths of Accuhaler, (ii) the very different name, packaging, shape, colour mechanism and mode of operation of the Glaxo and Sandoz inhalers and (iii) the fact that the evidence demonstrated that healthcare professionals would not make assumptions about the scope of a product’s marketing authorisations on the basis of colour. The judge concluded that there was no evidence that the use of the colour purple mispresented that the AirFluSal Forspiro had the same marketing authorisations as the Seretide Accuhaler.
In short
This decision underlines the difficulties of proving goodwill and passing off in colours and get up in the UK.
Case details at a glance
Jurisdiction: England & Wales
Decision level: High Court
Parties: Glaxo Wellcome UK Ltd & Anor v Sandoz Ltd & Ors
Date: 04 October 2019
Citation: [2019] EWHC 2545 (Ch)

